Marketing
Our competitiors and marketing strategies
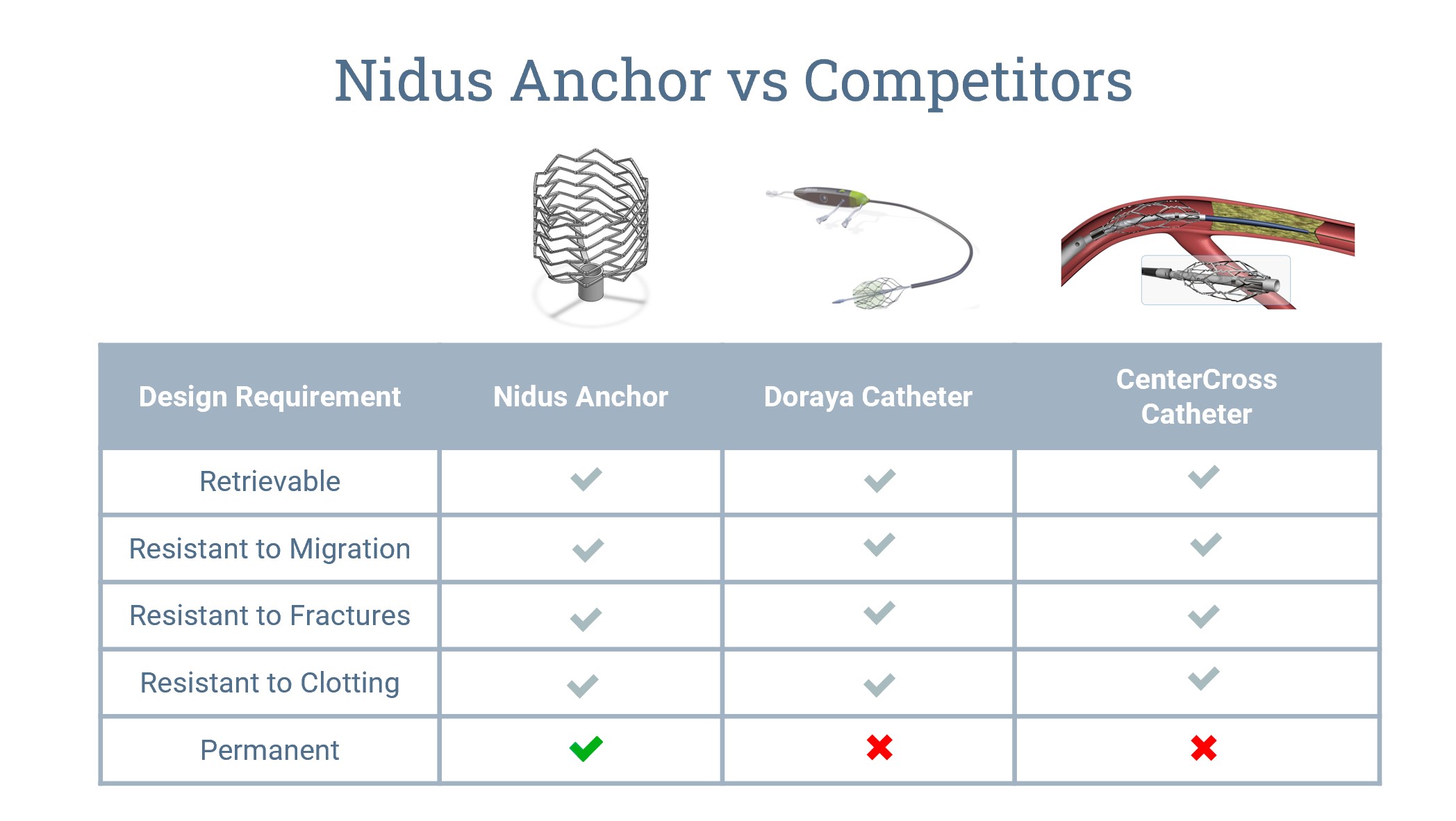
Our top two competitors would be the Doraya catheter which is placed in the IVC to relieve venous pressure for patients with acute heart failure and the CenterCross catheter which is used to center catheters in arteries that are completely blocked. Looking closer at competitors, it’s easy to see how similar characteristics such as retrievability and resistance to clotting are all accepted design criterias for all 3 devices. However, these are the bare minimum standards for stent devices to go into blood vessels. What makes our device stand out from the rest is that ours will be permanent in the body.
Device Comparison
The Doraya catheter is placed in the IVC for 12 hours or less and the CenterCross catheter is placed in an artery for only 5 ½ minutes. In order to make our device permanent, we have opted to go with an open-cell spiral z pattern for the stent. With our design, it allows for the stent to be more flexible, which is important in a vein where the compliance is very high. Additionally, our device will be made with thinner struts, which allows for less obstructions and higher stability once deployed. On the other hand, the other two competitors on this list have a braided, closed-cell pattern making their duration in the blood vessel shorter. While longer duration may not be their intended use, it is an important reminder that our device is solving kidney disease in a way that has never been done before. These are the closest competitors when it comes to looking at stent-like devices that anchor a catheter in a blood vessel.
Our prototype stems from other stent-like devices that anchor a catheter in the blood vessel such as the Doraya catheter and the CenterCross catheter. What makes the Nidus anchor stand out from the rest is that it will be permanent in the body due to its open-cell spiral z pattern for the stent. Whereas our competitors only allow for durations as long as 12 hours or less.
Marketing Strategies
Our target audience for this device will be primarily those over the age of 65. This demographic represents nearly 18% of the US population and are the most likely to have either heart failure or CKD. Additionally we would want to expand this market towards those prone to kidney diseases such as patients with diabetes. Because this device requires surgery to be inserted, we would most likely be looking to sell to hospitals rather than clinics or smaller establishments. Just to reiterate, this device can be used by those suffering from cardiac or renal diseases allowing us to tap into both of these markets. On average stents themselves cost near 6 to 800 dollars and just under 15,000 to be implanted which is significantly cheaper than dialysis which can amount to more than $72,000 per year. Each dialysis session lasts nearly 4 hours and patients have to go 3 times a week. Our device will be able to mitigate this time, providing a better quality of life to the patient.